Research Interests in Surface Chemistry
Mechanisms of Surface Hydrogenation Reactions
Our group conducts fundamental studies of the mechanisms of chemical reactions that take place on the surfaces of transition metals. These studies are motivated by a desire to gain a more detailed understanding of the surface chemical reactions that occur in heterogeneous catalysis. Spectroscopic methods are used to identify important surface intermediates and to measure the kinetics of surface chemical reactions. The technique of reflection absorption infrared spectroscopy (RAIRS) has proven to be particularly effective in identifying molecules on surfaces and in distinguishing between adsorbates with subtle differences in structure. We have worked to steadily improve the experimental capabilities of RAIRS and to understand various physical phenomena that influence the spectra.
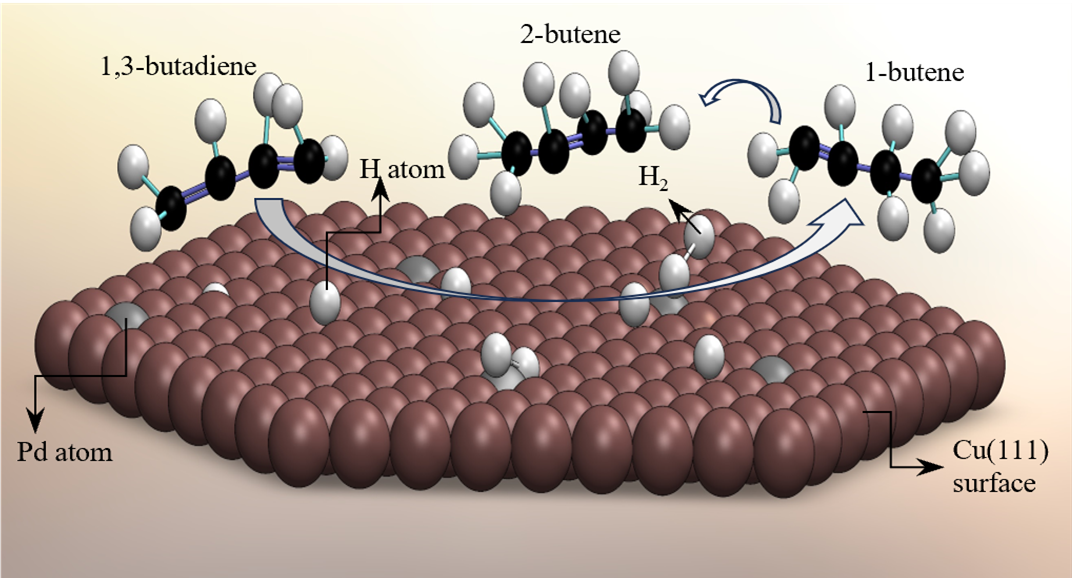
There is much current interest within the surface chemistry and catalysis communities in studies carried out under ambient pressure conditions. We have constructed an apparatus that allows single crystal surfaces to be prepared and characterized by standard ultra-high vacuum techniques and then to be transferred to a high-pressure cell for in situ characterization with RAIRS while reactions take place in the presence of gases at pressures up to one atmosphere. We have used this apparatus to study the complete and partial hydrogenation of acetylene, propyne, 1,3-butadiene, and acrolein. The method relies on the polarization dependence of the infrared radiation to observe only gas phase species or both gas and surface species. Using this method, we were able to simultaneously monitor the surface and gas phase species as C2H2(g) was first converted to C2H4(g) and then to C2H6(g) over Pt(111).
We are performing experiments on the mechanisms of hydrogenation reactions on a type of bimetallic surface known as single-atom-alloys (SAAs). An example of an SAA is a Cu(111) surface with an approximately 1% coverage of Pd atoms. Molecular hydrogen dissociates at the Pd sites to produce surface hydrogen atoms that then spillover to Cu sites. The resulting surface promotes low temperature selective hydrogenation reactions. The surfaces are prepared under ultra-high vacuum conditions according to well documented procedures. A variety of selective hydrogenation reactions are being studied. In each case, the objective is to obtain information on the identity of reaction intermediates and the activation barriers of elementary steps of the overall hydrogenation mechanism.
Surface Structure and Chemistry of the Boron-Rich Solids
The broad class of materials known as the boron-rich solids constitute a unique and fascinating group of compounds with many current and potential applications. These compounds are generally extremely hard with extremely high melting points. They include the metal borides of stoichiometry MBn, where n = 2, 4, 6, 12; solids based on B12 icosahedral units such as boron carbide, B12O2, B12P2, B12As2; as well as compounds of highly unusual stoichiometries such as YB66. Common to all these solids are extended networks of covalently bonded boron atoms. The bonding within the boron networks is generally described as electron deficient to convey the fact that they possess more bonding orbitals than can be filled by boron electrons. The unusual properties of these compounds make them uniquely well-suited to certain applications, such as the use of LaB6 and CeB6 as thermionic emitters, the use of YB66 as an X-ray monochromator, and the use of ZrB2(0001) substrates for the epitaxial growth of GaN thin films. Our group has published numerous studies on the surface structure and chemistry of single crystal surfaces of various boron-rich solids including LaB6(100), YB66(100), HfB2(0001), TaB2(0001), and ZrB2(0001). In 2008, we published a review of surface studies of metal hexaborides. We are currently funded by an NSF DMREF grant (2119308) to study the surface chemistry of MB2 thin films grown from M(BH4)4 gas-phase precursors.
Research currently funded by National Science Foundation Grants CHE 2102622 and DMREF 2119308.
National Science Foundation

National Science Foundation (NSF)